Organic Sales Soar to New Heights in 2020
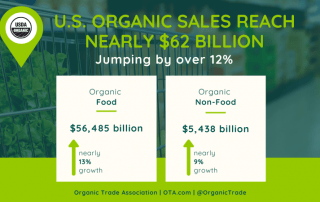
Source: Organic Trade Association Good news for organic sellers and partners of AgriSystems International. U.S. organic sales skyrocketed in 2020, increasing by a record 12.4 percent to $61.9 billion. Organic Products In High Demand For the first time, total sales of organic food and non-food products passed $60 billion. The recent 2021 [...]